Natco, 9 others chosen to supply COVID-19 drug
UN-backed MPP signs sublicence pacts
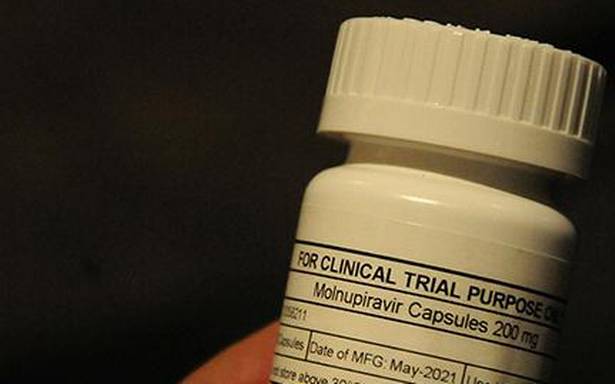
United Nations-backed public health organisation Medicines Patent Pool (MPP) has signed sublicence agreements with 10 generic drugmakers in India for the manufacturing of COVID-19 antiviral oral drug molnupiravir and supply in 105 low- and-middle-income countries (LMICs).
Natco, Strides, Biophore, SMS Pharmaceuticals, Laurus, Lupin, MSN, Arene Lifesciences, BDR and Optimus are thecompanies in a list of 27 generic drugmakers from 11 countries with whom MPP on Thursday said it has signed agreements.
The sublicenses are the result of a voluntary licensing agreement between MPP and MSD (Merck & Co) in October to facilitate affordable global access for molnupiravir, which MSD is developing in partnership with Ridgeback Biotherapeutics, it said.
The non-exclusive sublicenses allow the generic manufacturers to produce the raw ingredients for molnupiravir and/or the finished drug itself. The companies that were offered the sublicence demonstrated their ability to meet the requirements related to production capacity, regulatory compliance, and the ability to meet international standards for quality-assured medicines, MPP said. Of the 27 firms, five will focus on producing the raw ingredients, 13 both raw ingredient and the finished drug, while nine companies will produce the finished drug.
Neither MSD, Ridgeback Biotherapeutics nor Emory University, which invented the drug, will receive royalties from sales of molnupiravir from the sublicencees while COVID-19 remains classified as a Public Health Emergency of International Concern by WHO, a communication on MPP website said.
Natco Pharma said under the agreement it can manufacture and sell molnupiravir capsules 200 mg for Indian market, which will be sold under brand name Molnunat. The agreement also allows it to expand access to COVID-19 medicines in 105 countries in generic name. The company can set its own price for the generic products it produces, paying a royalty on sales to MSD, Natco said in a release.
Biophore India Pharmaceuticals said development of the product is complete and it is gearing up for launch based within the next week. The licence enables it to launch the product within India as well as export to 104 other countries. For the Indian market, it will launch the drug next week at ₹1,500 for a pack of 40 capsules, the company said.
Strides Pharma Science and subsidiary Universal Corporation Ltd. (Kenya) have entered into the sub‐licence partnership under which they will make the product at their WHO PQ facilities in India and Nairobi. MD and CEO of Strides Pharma Science R. Ananthanarayanan said “while our 200mg strength (molnupiravir) has been launched in India under Stripiravir brand, this partnership with MPP will enable us to commercialise the 400mg dose along with 200mg dose for global markets.”
Source: Read Full Article